ا
في الأول من أغسطس/آب 2025، أصدرت محكمة العدل التابعة للاتحاد الأوروبي حكماً نهائياً يؤيد حكمها الصادر عام 2022.
هذا يعني إزالة تصنيف مسحوق ثاني أكسيد التيتانيوم (TiO₂) كمسرطن. يُنهي هذا القرار جدلاً علمياً وتنظيمياً استمر قرابة عقد من الزمان، وأثّر على العديد من الصناعات حول العالم.
ثاني أكسيد التيتانيوم
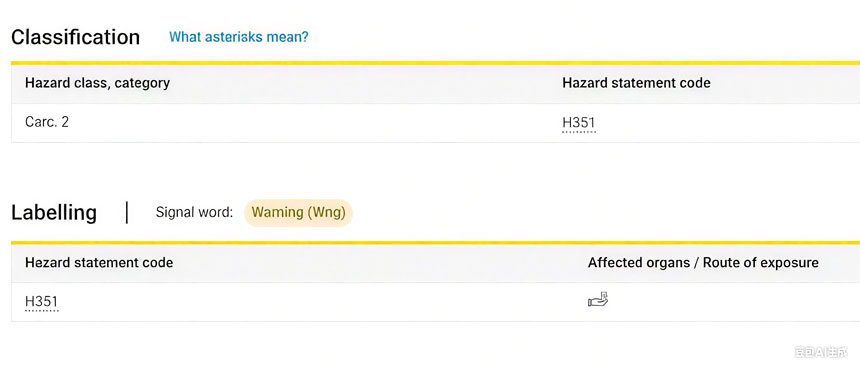
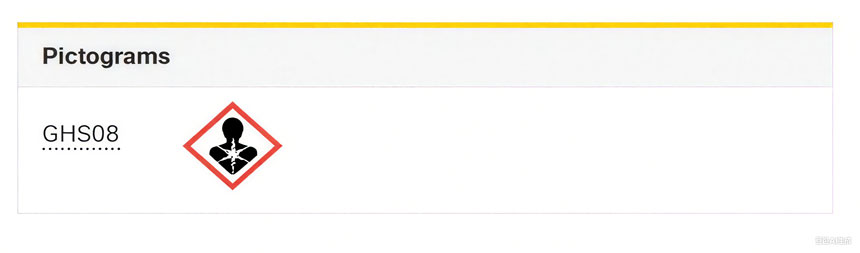
يُستخدم مسحوق ثاني أكسيد التيتانيوم (TiO2) على نطاق واسع في الطلاءات والمستحضرات الصيدلانية والأغذية نظرًا لقدرته الممتازة على إخفاء الشوائب وبياضه. في عام ٢٠١٦، قدمت الوكالة الفرنسية لسلامة الأغذية والصحة البيئية والمهنية (ANSES) مقترحًا إلى الوكالة الأوروبية للمواد الكيميائية (ECHA) يطلب تصنيف مسحوق ثاني أكسيد التيتانيوم "كمادة مسرطنة عن طريق الاستنشاق". في عام ٢٠١٧، اعتمدت لجنة تقييم المخاطر (RAC) التابعة للوكالة رأيًا يدعم تصنيفه "كمادة مسرطنة مشتبه بها من الفئة ٢". في أكتوبر ٢٠١٩، اعتمدت المفوضية الأوروبية اللائحة المفوضة (DE) ٢٠٢٠/٢١٧، التي تُصنف رسميًا ثاني أكسيد التيتانيوم في شكل مسحوق (١٪ أو أكثر من الجسيمات ≤ ١٠ ميكرون) كمادة مسرطنة مشتبه بها من الفئة ٢، وتشترط استخدام ملصق تحذيري: "H351: قد يُسبب السرطان عن طريق الاستنشاق".
التصنيف والوسم الحاليين هما كما يلي:
الجدول الزمني لتصنيف ثاني أكسيد التيتانيوم
|
سنة
|
حدث
|
تأثير
|
|
2016
|
تقترح ANSES الفرنسية تصنيف TiO₂ كـ
مادة مسرطنة
|
أثار القلق التنظيمي الأولي
|
|
2017
|
تدعم لجنة الأنشطة الإقليمية التابعة لوكالة المواد الكيميائية الأوروبية تصنيف الفئة 2
|
مصادقة اللجنة العلمية
|
|
2019
|
اعتمدت مفوضية الاتحاد الأوروبي اللائحة المفوضة (الاتحاد الأوروبي) 2020/217
|
تم تطبيق ملصقات التحذير الإلزامية H351
|
|
2022
|
المحكمة العامة للاتحاد الأوروبي تلغي التصنيف
|
التحدي القانوني ينجح
|
|
2025
|
محكمة العدل الأوروبية تؤكد الإلغاء
|
الحل النهائي للنزاع
|
الأساس العلمي للقرار
استند حكم محكمة العدل التابعة للاتحاد الأوروبي إلى ثلاثة اعتبارات علمية رئيسية:
1. آلية العمل:
واتفقت المحكمة على أن أورام الرئة التي لوحظت في الدراسات التي أجريت على الفئران كانت ناجمة عن التحميل الزائد للجسيمات، وليس عن المسرطنة الذاتية لثاني أكسيد التيتانيوم نفسه.
2. الاستقراء على البشر:
وخلصت المحكمة إلى عدم وجود أدلة كافية لإثبات أن دراسات استنشاق الفئران يمكنها التنبؤ بشكل موثوق بخطر الإصابة بالسرطان لدى البشر، وخاصة عند مستويات التعرض النموذجية.
3. تفسيرات أخرى:
وجاء في الحكم أن التأثيرات التي لوحظت ربما تكون ناجمة عن التهاب ناجم عن تراكم الجسيمات، وليس عن سمية كيميائية.
التأثير المباشر على الصناعة
سيوفر هذا القرار تخفيفًا كبيرًا للعديد من الصناعات:
الدهانات والطلاءات
:
إزالة ملصقات التحذير الموجودة على معظم الطلاءات المعمارية والصناعية
البلاستيك
:
تخفيف عبء التصنيف على تغليف الأغذية والمنتجات الاستهلاكية
مستحضرات التجميل:
يمكن الآن تسويق واقيات الشمس ومستحضرات التجميل الملونة دون تحذيرات من المواد المسرطنة
صناعة الأغذية:
استعادة الثقة في استخدام TiO₂ (E171) في تطبيقات الأغذية
التأثير التنظيمي العالمي
ورغم أن قرار الاتحاد الأوروبي ملزم للدول الأعضاء، فإن المناطق الأخرى قد تتفاعل بشكل مختلف:
نحن:
تحافظ إدارة السلامة والصحة المهنية (OSHA) على التصنيف الحالي غير المسرطن
كندا:
تستمر وزارة الصحة الكندية في مراقبة الأدلة الناشئة
آسيا:
لم تعتمد معظم الأسواق أبدًا التصنيف الاحترازي للاتحاد الأوروبي
المنتجات التي تحتوي على ثاني أكسيد التيتانيوم
يظل TiO₂ ضروريًا في العديد من المنتجات الشائعة:
|
فئة المنتج
|
وظيفة TiO₂
|
التركيز النموذجي
|
|
الدهانات
|
معتم/أبيض
نير
|
15-25%
|
|
البلاستيك
|
مثبت الأشعة فوق البنفسجية
|
0.5-5%
|
|
واقيات الشمس
|
فلتر الأشعة فوق البنفسجية
|
2-10%
|
|
طعام (E171)
|
ملون
|
0.1-1%
|
|
ورق
|
مُبيّض
|
2-8%
|
يُقدّم الحكم النهائي لمحكمة العدل الأوروبية توضيحًا بالغ الأهمية بشأن سلامة ثاني أكسيد التيتانيوم، مؤكدًا موقف العديد من العلماء والمصنّعين. ورغم أهمية اليقظة التنظيمية، يُمكّن هذا الحكم الصناعة من الاستفادة من خصائص ثاني أكسيد التيتانيوم القيّمة دون تحذيرات غير ضرورية من احتمالية تسببه في السرطان.
التعليمات
س1: ما هو مسحوق ثاني أكسيد التيتانيوم TiO2؟
أ: ثاني أكسيد التيتانيوم هو أكسيد التيتانيوم الطبيعي الذي يستخدم كصبغة بيضاء ومادة مضافة وظيفية في العديد من التطبيقات الصناعية والاستهلاكية.
س2: ما هي الصبغات الشائعة التي تحتوي على ثاني أكسيد التيتانيوم؟
أ: تحتوي العديد من الصبغات المهمة على ثاني أكسيد التيتانيوم:
ثاني أكسيد التيتانيوم (TiO₂): أصباغ ثاني أكسيد التيتانيوم النقية
أصباغ لؤلؤية
:في كثير من الأحيان يتم استخدام الميكا المغطاة بثاني أكسيد التيتانيوم لتحقيق تأثيرات قزحية اللون
الأصباغ المركبة: تتحد العديد من الأصباغ مع ثاني أكسيد التيتانيوم مع ملونات أخرى
س3: هل ثاني أكسيد التيتانيوم آمن للاستخدام في مستحضرات التجميل؟
ج: إنه آمن للاستخدام المقصود منه. ويؤكد قرار صادر عن الاتحاد الأوروبي بوضوح عدم وجود دليل على أنه يسبب السرطان عند ملامسته للجلد.
س4: هل سيتم إعادة الموافقة على ثاني أكسيد التيتانيوم الصالح للأكل (E171) في الاتحاد الأوروبي؟
أ: في حين أن حكم المحكمة لا يعيد العمل بالمادة E171 تلقائيا، فإنه يزيل الأساس العلمي لحظرها في عام 2022، وهو ما قد يمهد الطريق لإعادة النظر.
س5: كيف ينبغي للشركات المصنعة تحديث ملصقات منتجاتها؟
أ:
1. ينبغي على الشركات: إزالة تحذير H351 على الفور من المنتجات التي تحتوي على ثاني أكسيد التيتانيوم.
2. مراجعة صحائف بيانات السلامة للحصول على تحديثات الامتثال.
3. استشر فريقك القانوني فيما يتعلق بمتطلبات وضع العلامات الانتقالية.
بقلم ليزا تشين، الحاصلة على درجة الدكتوراه، خبيرة سلامة المواد بخبرة 15 عامًا في تقييم المخاطر الكيميائية. آخر تحديث: أغسطس 2025











 +86 13965049124
+86 13965049124
 العربية
العربية  English
English français
français русский
русский italiano
italiano español
español português
português 한국의
한국의 ไทย
ไทย Tiếng Việt
Tiếng Việt






